WELCOME TO CLIMAFRICA-CRO
CliMAfrica-CRO is a Contract Research Organization (CRO) established in Burkina Faso (Western Africa) that supports clinical research across Africa. The main objective of CliMAfrica-CRO is to provide quality services in clinical research in Africa. CliMAfrica-CRO has expertise in monitoring of clinical studies, management and coordination of clinical studies, management of data generated in clinical studies and training of personnel in various fields of clinical research. All of our actions are guided by a clear vision and values.
All services provided by CliMAfrica-CRO are performed in accordance with the sponsor SOP, ICH/GCP and applicable regulatory requirements.
ABOUT US
OUR VALUES

- More hands and minds put together allow better results. CliMAfrica-CRO is composed of men and women with track records in monitoring, management or coordination of clinical research studies in Africa.
- Promote (win-to-win) reliable partnerships with pharmaceutical companies, academia sponsors, major CROs for success.
- Work for the satisfaction of customers by providing high quality of services and surpassing client expectations
- Ethics, integrity, honesty, professionalism and hard work are our day to day leitmotiv
- Always keep in mind that trial participants safety and integrity and quality of data are critical for any clinical study
OUR SKILLS

- More hands and minds put together allow better results. CliMAfrica-CRO is composed of men and women with track records in monitoring, management or coordination of clinical research studies in Africa.
- Promote (win-to-win) reliable partnerships with pharmaceutical companies, academia sponsors, major CROs for success.
- Work for the satisfaction of customers by providing high quality of services and surpassing client expectations
- Ethics, integrity, honesty, professionalism and hard work are our day to day leitmotiv
- Always keep in mind that trial participants safety and integrity and quality of data are critical for any clinical study
OUR VISION

Our vision is to be the leading Contract Research Organization in Africa. To achieve this vision we are always oriented to looking for our customer’s satisfaction.
Our Services
we provide best services for you.
Monitoring of Clinical Trials
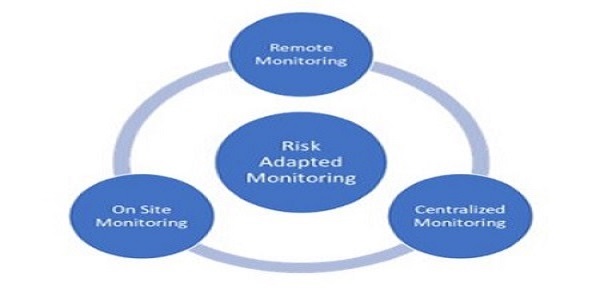
-
- Site Qualification visit
- Site initiation visit
- Routine monitoring visits including SDV activities, assessment of site compliance to protocol and SOP, investigational products review and accountability, lab samples review and laboratories review, review of essential documents, review of site facilities, …
- Site Close out visit
- Site management
- Pharmacovigilance
- Data management
- Support to audits and inspections
Training & Capacity Building
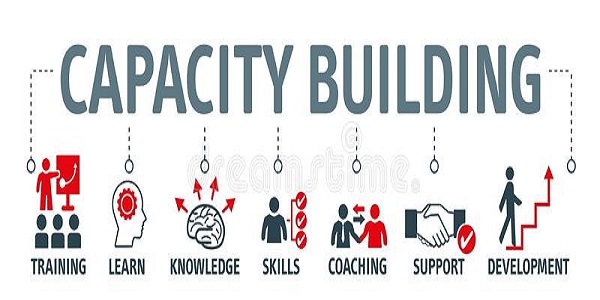
Common difficulty of clinical trials implementation in Africa is inadequacy of qualified human resources. We provide a wide training program in clinical research including:
-
- Good Clinical Practice (GCP)
- Good Clinical Laboratory Practice (GLP)
- Ethics in clinical research
- CRAs training
Clinical Trials Management

-
- Country feasibility, investigator and site selection
- Assistance in the design, planning and implementation of clinical research
- Regulatory submission in African countries on behalf of the sponsor
- Development and / or review of study documentation including protocol, ICF, CRF, diary cards, …
- Development and / or review of documents including Study Manual, Monitoring Plan, Trial master file, Investigator site file, monitoring tools
- Planning, preparation and coordination of investigator’s meetings
- Planning, preparation and coordination of monitoring activities
- Coordination of shipment of biological samples in accordance with IATA and country regulations
- Coordination of shipment of study documents, supplies, …
- Staff outsourcing (Junior CRA, Senior CRA, unblinded CRA Project manager, CTA, study coordinator, … )
Data Management Solutions

In collaboration with our partner NOYMED, we offer provides scalable clinical trial solutions to pharmaceutical, biotechnology and medical device industries worldwide.Our experts’ experiences are focused on following EDC technology: Medidata rave, Open Clinica, Medrio, Oracle.
-
- Data management services for paper- based and electronic data capture (edc) based trials
- Case Report Form (CRF), electronic and/or paper
- Data management plan (dmp) creation
- Quality Control (QC) sampling for paper-based studies
- Data entry and validation
- Data cleaning
- Database lock
- Data reconciliation and/or coding
- Archiving of study databases and related documents
- Close-out audit for closing of study trial in EDC
- Query management
Therapeutic Areas
We served academical and pharmaceutical industry in a wide variety of therapeutic areas. A summary of therapeutic areas is summarised as below:
- Immunology
- Infectious diseases
- Medical devices
- Vaccine
- Haematology
- Orthopaedics
- Cardiovascular
- Pulmonology
- Diabetes
- Metabolic diseases
- Rheumatology
- Gastroenterology
Request For a Quote
Fill in the fields of the form and click on the send button to send us your request.
Fields marked with an * are required
People Who work with us







